AlN is a stable covalent bond compound with hexagonal wurtzite structure and no other homomorphs. Its crystal structure is composed of the AlN4 tetrahedron produced by the conversion of aluminum atoms and adjacent nitrogen atoms. The space group is P63mc, belonging to the hexagonal system.
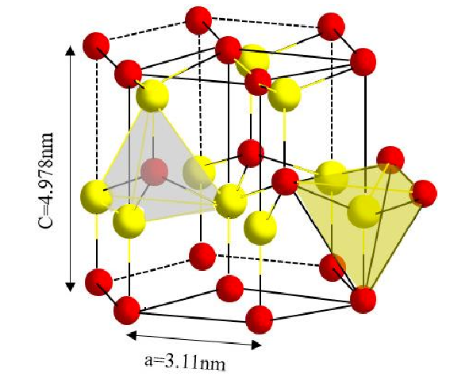
schematic diagram of AlN crystal structure
The main characteristics of AlN ceramics
(1) High thermal conductivity, 5-10 times that of alumina ceramics;
(2) The coefficient of thermal expansion (4.3×10-6/℃) matches the semiconductor silicon material (3.5-4.0×10-6/℃);
(3) Good mechanical properties;
(4) Excellent electrical performance, with very high insulation resistance and low dielectric loss;
(5) Multi-layer wiring can be carried out to achieve high density and miniaturization of packaging;
(6) Non-toxic, conducive to environmental protection.
Various Factors Affecting Thermal Conductivity of AlN Ceramic Substrate
At 300K, the theoretical thermal conductivity of AlN single crystal material is as high as 319W/(m·K), but in the actual production process, due to the purity of the material, internal defects (dislocation, porosity, impurities, lattice distortion), grain orientation and sintering process and other factors, its thermal conductivity will also be affected, often lower than the theoretical value.
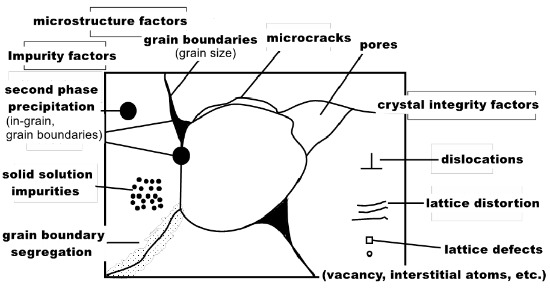
factors affecting thermal conductivity of AlN ceramics
Effect of microstructure on thermal conductivity
The heat conduction mechanism of single crystal AlN is phonon heat transfer, so the thermal conductivity of AlN substrate may be mainly affected by the scattering control of crystal boundary, interface, second phase, defect, electron and phonon itself. According to the lattice solid vibration theory, the relation between phonon scattering and thermal conductivity λ is as follows:
λ=l/3cv, where c is the heat capacity, v is the mean speed of phonons, and l is the mean free path of phonons.
From the above formula, it can be seen that the thermal conductivity λ of aluminum nitride is proportional to the mean free path l of phonons, and the larger l is, the higher the thermal conductivity is. From the perspective of microstructure, the interaction between phonons and phonons, the interaction between phonons and impurities and grain boundary defects will cause scattering, which will affect the mean free path of phonons, and thus affect its thermal conductivity.

The microstructure of AlN has a great influence on its thermal conductivity. In order to obtain aluminum nitride substrate with high thermal conductivity, it is necessary to minimize the defects of aluminum nitride crystals and the content of impurities.
Effect of oxygen impurity content on thermal conductivity
Studies show that AlN has a strong affinity with oxygen and is easy to oxidize, resulting in an alumina film on its surface. Due to the dissolution of oxygen atoms in Al2O3, it replaces the position of nitrogen atoms in AlN, resulting in an aluminum vacancy and forming an oxygen defect. The specific reaction is as follows:
Al2O3→2Al+3O, where ON is the position where oxygen atoms replace nitrogen in the aluminum nitride lattice, and VAl is the vacancy of aluminum.
The resulting aluminum vacancy scatters the phonons, resulting in a decrease in the mean free path of the phonons, so the thermal conductivity of the AlN substrate also decreases.
It is concluded that the types of defects in AlN lattice are related to the concentration of oxygen atoms.
When the oxygen concentration is lower than 0.75%, the oxygen atoms are evenly dispersed in the AlN lattice, replacing the nitrogen atoms in the AlN, and the aluminum holes are formed.
When the oxygen concentration is not less than 0.75%, the position of the aluminum atoms in the AlN lattice will change, and the aluminum vacancy will disappear, resulting in octahedral defects.
When the concentration of oxygen atoms is higher, the lattice will produce many types, inversion domains, oxygen-containing layer faults and other extension defects. Taking thermodynamics as the starting point, it is found that the amount of oxygen in the aluminum nitride lattice is affected by the Gibbs free energy of aluminate reaction |ΔG°|. The greater the |ΔG°|, the less oxygen in the aluminum nitride lattice, and thus the higher the thermal conductivity.
It can be seen that the thermal conductivity of AlN is seriously affected by oxygen impurities, and the existence of oxygen impurities is an important reason for the decrease of its thermal conductivity.
Suitable Sintering Additives Ensure to Improve the Thermal Conductivity
In order to improve the thermal rate of AlN, the required sintering aid is usually added during sintering to reduce the sintering temperature and remove oxygen in the lattice, thus achieving the purpose of increasing the thermal conductivity of AlN.
At present, more attention is paid to the addition of multi-component composite sintering additives. Experiments have found that when adding composite sintering AIDS Y2O3-Li2O, Y2O3-CaC2, Y2O3-CaF2 and Y2O3-Dy2O3 to AlN, relatively dense AlN samples with less oxygen impurities and second phase can be obtained.

The suitable sintering additives of the composite system can achieve low sintering temperature of AlN and effectively purify the grain boundary, and obtain AlN with high thermal conductivity.




























